A medical coating
& device solutions company
Lynk Coating is designed
to solve surgical complications
Lynk Coating can be applied to a variety of medical devices with different materials,
providing solutions that enhances the safety and precision of surgeries
in a wide range of fields for both medical professionals and patients.
PUBLICATIONS




NEWS
Advances in surgical techniques and the growing elderly population have led to an increasing number of medical devices—such as catheters and stents—being implanted inside the human body. Over time, however, these implanted devices accumulate various biological contaminants, including proteins, cells, and bacteria. This buildup can trigger complications such as infections, inflammation, thrombosis, and calcification. These issues cause patient discomfort, degrade device performance, and often result in repeated procedures, ultimately imposing a significant healthcare burden.
In addition, endoscopes used during surgery frequently fog up due to the high humidity inside the human body, and blood often adheres to their surface. As a result, surgeons must repeatedly withdraw the endoscope for cleaning before resuming the procedure. In fact, approximately 20% of total surgery time is reportedly spent on endoscope cleaning, and some operating rooms even assign dedicated staff for this task.
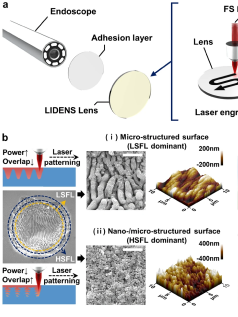
To solve these problems, a startup named Lynk Solutec has developed an innovative coating technology. The company has created a nano–lubricant-film–based anti-biofouling coating called LYNK Coating, inspired by the surface structure of the butterwort plant. This technology suppresses biological adhesion purely through physical and chemical control—without using drugs—and can be uniformly applied to diverse materials such as polymers, metals, and ceramics, as well as to complex device geometries. On the company’s website, detailed demonstration videos show the performance of Clean View, a non-fogging, blood-repellent endoscope coating, along with the LYNK Solution anti-biofouling coatings and release agents designed to prevent surface contamination.
Lynk Solutec’s endoscopic products have already received Class I regulatory approval and are undergoing clinical validation at major hospitals. The company also plans to launch coated catheters, ureteral stents, and other product lines once regulatory processes are complete.
Lynk Solutec was founded in 2020 by Professor Jungmook Seo, who earned his PhD in electrical and electronic engineering from Yonsei University and later served as a postdoctoral fellow at Harvard Medical School, and Dr. Yeontaek Lee, who studied materials science and electrical engineering at Yonsei University. One of the company’s strengths is its fully integrated development system—from research and product development to regulatory approval and mass manufacturing.
The company raised a ₩1 billion seed investment from Daily Partners and the Yonsei University Technology Holdings in 2022, followed by selection for the TIPS program. In 2025, it secured ₩1.5 billion in Pre-A funding from POSTECH Holdings and the Korea Technology Finance Corporation, demonstrating strong validation of its technology. Lynk Solutec will present its vision for global expansion at the PR Day event hosted by POSTECH Holdings on December 5.
What problem is Lynk Solutec solving, and how?
Implanted medical devices develop surface contamination over time—proteins, bacteria, and other bio-substances—that lead to infections, inflammation, thrombosis, and calcification. These complications degrade device performance and often require additional surgical procedures.
To address these issues fundamentally, we developed LYNK Coating, a nano–lubricant-film–based anti-biofouling technology.
Inspired by the surface structure of the butterwort plant, the coating forms an ultrathin lubricating layer that prevents adherence from millimeter-scale contaminants down to microscopic proteins. Since it suppresses adhesion without using drugs, it offers high safety and long-term stability. It can be uniformly applied to polymers, metals, ceramics, and even devices with complex geometries. Ultimately, it can extend device lifespan, reduce complications, and lower healthcare costs.
What are the technical advantages of your products and services?
Our core technology is LYNK Coating, applied across a wide range of implanted medical devices—from the Clean View endoscopic coating to silicone catheters and ureteral stents.
The technology fundamentally blocks biological adhesion without drugs, ensuring both durability and safety. It enables uniform coating across diverse materials and complex structures, and can be applied easily within GMP manufacturing environments.
Our endoscopic products have already obtained Class I approval and are undergoing real-world clinical testing in major hospitals. Our implantable product lines—including ureteral stents, catheters, and ptosis-correction sutures—are now progressing through regulatory stages.
What is the target market size, key customers, and the business model?
The global medical device market reached roughly KRW 726 trillion in 2023, with the implantable device segment alone accounting for about KRW 168 trillion. We are initially targeting medium- to long-term implantable devices that frequently contact blood or bodily fluids and plan to address a market of approximately KRW 55 trillion by 2030.
Our key customers include medical device manufacturers, tertiary hospitals, and public procurement agencies.
Our business model has two phases:
- Phase 1: Direct sales of coated medical devices to validate the technology and generate initial revenue.
- Phase 2: Expansion into coating-technology licensing, coating-solution and equipment supply, and OEM manufacturing.
Through clinical validation and procurement-based pilot programs, we aim to enter the market and scale globally with our technological, quality, and GMP-based manufacturing capabilities.
What achievements has Lynk Solutec made so far?
Lynk Solutec secured a ₩1 billion seed investment from Daily Partners and Yonsei University Technology Holdings in 2022, followed by TIPS selection (₩500 million). In 2025, the company raised ₩1.5 billion in Pre-A funding from POSTECH Holdings and the Korea Technology Finance Corporation.
With these investments, we accelerated R&D and successfully built a portfolio of five domestic patents, two foreign patent applications, five coating pipelines, and four product lines.
What is the team structure, and what makes it competitive?
Lynk Solutec is a technology-driven medical device startup founded by Professor Jungmook Seo—PhD in electrical and electronic engineering and former postdoctoral fellow at Harvard Medical School—and Dr. Yeontaek Lee, who specialized in materials science and electrical engineering at Yonsei University.
We operate an in-house system that connects research, product development, regulatory approval, and mass manufacturing. This allows us to translate ideas into actual medical devices quickly. Ongoing collaborations with major hospitals—including Boramae Hospital, Korea University Ansan Hospital, and Severance Hospital—provide continuous clinical validation and field insights.
This integrated team structure of research, clinical testing, and manufacturing enables us to rapidly mitigate risks in clinical approval and mass production.
How has POSTECH Holdings supported Lynk Solutec?
POSTECH Technology Holdings has gone beyond investment by facilitating partnerships with hospitals and offering ongoing support. Through their consulting for follow-on fundraising, we strengthened our IR capabilities and refined our business direction.
What message would you like to deliver to investors at the upcoming PR Day Demo Day?
This Demo Day comes at a pivotal moment—as we prepare for FDA and CE regulatory submissions and global commercialization.
Our coating technology solves infection, thrombosis, and calcification challenges in implantable devices without drugs. Our flagship product, Clean View, has already secured regulatory approval and adoption in hospital pilot programs, generating real revenue. Our founding team includes two PhD-level experts, supported by GMP-based manufacturing infrastructure and a strong network of clinical advisors, enabling us to minimize risks in clinical, regulatory, and production stages.
With our technology validated and market traction confirmed, now is the ideal time for investors to join us as we transition into the second stage of our business model and accelerate global growth.
- 2025-11-11
Lynk Solutec has been officially designated as an Innovative Product by the Public Procurement Service (PPS), marking a significant milestone for the company’s entry into the public procurement market and the expansion of its technology commercialization. This designation represents national recognition of the company’s technological capabilities and innovation.
The designated product is C.E.V. (Innovative Product Name: Endoscope Connector for Securing Clear Vision), a medical device component designed to prevent contamination from bodily fluids and foreign substances during endoscopic procedures, while ensuring clear visibility. Powered by Lynk Solutec’s proprietary coating technology, the product enhances procedural efficiency for medical professionals and improves patient safety.
This recognition is expected to strengthen Lynk Solutec’s competitiveness in the public procurement market while creating new opportunities for collaboration with medical and public institutions.
Moving forward, Lynk Solutec will continue to advance next-generation coating technologies and drive consistent quality innovation, with the goal of delivering tailored coating solutions optimized for clinical applications both domestically and globally.


- 2025-09-04
Lynk Solutec Inc. participated in the “Lab-Thursday Talk” program hosted by Ewha Womans University Medical Center, presenting its latest medical coating technologies. The session was led by Dr. Yeontack Lee, CEO of Lynk Solutec, who introduced the company’s innovative coating solutions, their applications in medical devices, and potential directions for future collaborative research.
Lab-Thursday Talk is a regular program designed to foster collaboration between clinical and basic researchers and industry partners. Having been held more than 30 times, it has become a key platform for sharing research expertise and exploring opportunities for joint R&D projects.
Through this presentation, Lynk Solutec highlighted the innovation of its endoscopic coating technology and other medical device applications, while seeking opportunities for clinical collaboration and co-development. Moving forward, the company will continue to strengthen its research and development capabilities, expand its collaboration network with domestic and international medical institutions, and provide optimized coating solutions tailored to clinical needs.


- 2025-09-02
Lynk Solutec, a startup specializing in surface coatings for medical devices, announced on the 12th that it has secured 1.5 billion KRW in Pre-Series A funding. The investment round was jointly led by POSTECH Holdings and the Korea Technology Finance Corporation (KOTEC).
Lynk Solutec is developing a nano oil-film-based non-adhesive coating technology aimed at reducing complications such as infections, inflammation, and thrombosis associated with implantable medical devices. This technology suppresses the adhesion of proteins, immune cells, and bacteria to device surfaces, and helps maintain stable performance over extended periods after implantation.
According to the company, “The technology is applicable to a wide range of medical devices including endoscopes, ureteral stents, catheters, and ophthalmic silicone tubes.” A product utilizing this coating for endoscopic applications was awarded Innovation Awards in both the Human Security and Digital Health categories at CES 2024.
Lynk Solutec is a spin-off from the BioLogical Interfaces & Sensor Systems Lab at Yonsei University. The company is currently engaged in joint research and commercialization projects with multiple hospitals and medical device manufacturers. It is also developing follow-up products for use in ophthalmology and urology.
Jungmok Seo, CEO of Lynk Solutec, stated, “This investment will enable us to begin full-scale mass production and sales of our coated endoscopic products,” adding, “At the same time, we will accelerate our global strategies, including portfolio expansion, FDA approval, and market entry in the Middle East.”
- 2025-06-12
- 머니투데이

